28th September 2023, Dr Chee L Khoo

Five years ago, we previewed the arrival of the twincretins. These are agents which contain both incretins, GLP1 and GIP. Back then, agent LY3298176 was shown to cause significant weight loss and reduction in glucose. Two years ago, agent LY3298176 finally had a name – tirzepatide. Two years ago, we looked briefly at the clinical trials comparing tirzepatide with various glucose lowering agents. The efficacy of tirzepatide in glucose lowering was just mind boggling and we have been waiting for its arrival ever since. Well, it’s finally here.
Tirzepatide is one molecule which binds to a GLP1 receptor at one end and a GIP receptor at the other end. It is an imbalanced dual agonist in favour of GIP over GLP1-RA activity as the molecule shows equal affinity for the GIP receptors compared with native GIP but binds the GLP-1 receptors with 5-fold weaker affinity than native GLP-1. Weaker affinity for GLP1 receptors may mean less GI side effects. Tirzepatide contains a C20 unsaturated di-acid acyl chain to affect albumin binding, and the overall properties of the molecule enable once-weekly dosing.
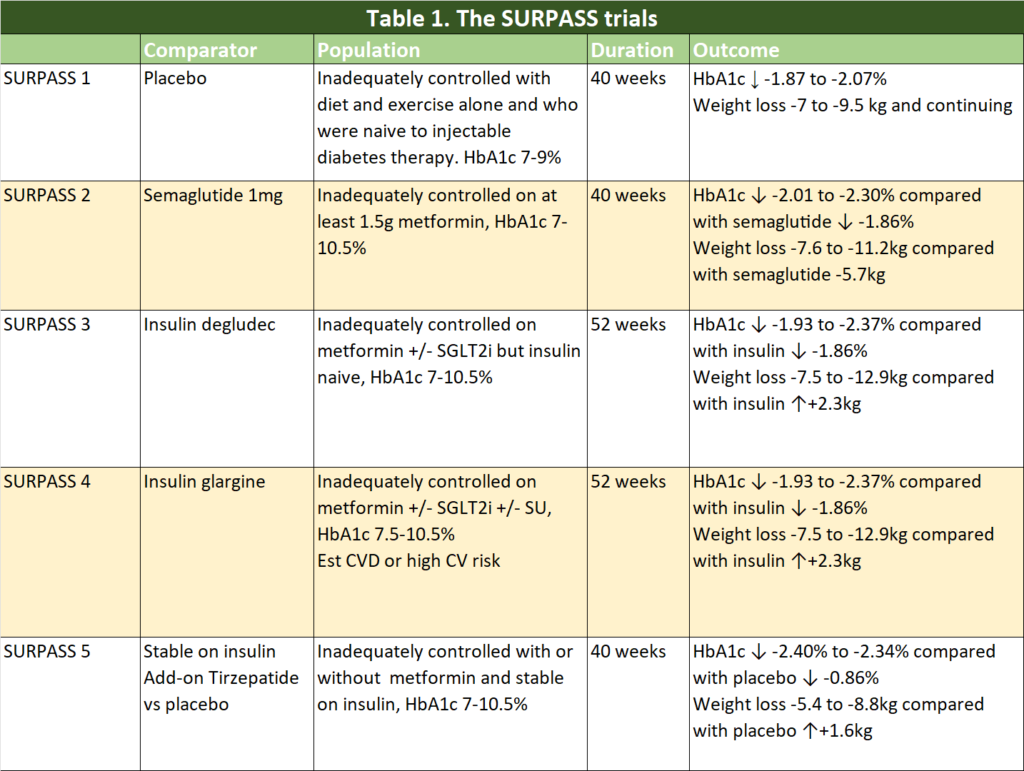
There have been five Phase 3 Clinical Trials comparing tirzepatide with placebo and with what we currently used to improve glycaemic control in our patients with type 2 diabetes (T2D) – semaglutide and basal insulins. Many of the patients were already on a multitude of glucose lowering agents and tirzepatide were added to existing regimen. In some of the trials, patients recruited were at high risk of cardiovascular disease.
Table 1 summarises the clinical trials that has published thus far.
We are all comfortable with the new user-friendly injectable pens and it certainly makes the uptake of injectables by patients so much easier. Suddenly, needle phobia seems a rare phenomenon even amongst patients who don’t have diabetes! We all know that supplies for GLP1-RA are limited and stocks for semaglutide are getting low again and soon we will be back to where we were 12 months ago when all stocks just dried up. Obviously, one of the reasons has to do with the incredible demand for the efficacious agent. Another reason is actually to do the difficulty in manufacturing process. For the time it takes to produce a self-injectable pen, one can produce 20 old style tirzepatide vials. This is exactly what Lilly Pharmaceuticals have done. Australia is the first country in the world to receive and use tirzepatide in vials.
For now, tirzepatide comes with 6 doses – 2.5mg, 5mg, 7.5mg, 10mg, 12.5mg and 15mg in one use 0.5ml vial. Each prescription comes with 4 vials for the month and a separate packet of 1ml insulin syringes with standard insulin type needles already connected. Patients can be easily taught to draw up the solution and self inject.
Tirzepatide is TGA approved for glucose lower in patients with T2D and is NOT TGA approved for weight loss. It is available as a private script and NOT on the PBS. The price is a pretty steep but for some patients, the efficacy of tirzepatide may be worth the cost. Further, for some patients initiation of tirzepatide may mean avoidance or delay of insulin therapy. Of course, the concomitant weight loss may result in diabetes remission. At the very least, it will improve insulin resistance and other metabolic derangements.
References:
- Rosenstock J, Wysham C, Frías JP, Kaneko S, Lee CJ, Fernández Landó L, Mao H, Cui X, Karanikas CA, Thieu VT. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021 Jul 10;398(10295):143-155. doi: 10.1016/S0140-6736(21)01324-6
- Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, Liu B, Cui X, Brown K; SURPASS-2 Investigators. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N Engl J Med. 2021 Aug 5;385(6):503-515. doi: 10.1056/NEJMoa2107519.
- Ludvik B, Giorgino F, Jódar E, Frias JP, Fernández Landó L, Brown K, Bray R, Rodríguez Á. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021 Aug 14;398(10300):583-598. doi: 10.1016/S0140-6736(21)01443-4.
- Del Prato S, Kahn SE, Pavo I, Weerakkody GJ, Yang Z, Doupis J, Aizenberg D, Wynne AG, Riesmeyer JS, Heine RJ, Wiese RJ; SURPASS-4 Investigators. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021 Nov 13;398(10313):1811-1824. doi: 10.1016/S0140-6736(21)02188-7.
- Dahl D, Onishi Y, Norwood P, Huh R, Bray R, Patel H, Rodríguez Á. Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial. JAMA. 2022 Feb 8;327(6):534-545. doi: 10.1001/jama.2022.0078
