25th November 2023, A/Prof Chee L Khoo
Reproductive genetic carrier screening for cystic fibrosis (CF), spinal muscular atrophy (SMA) and fragile X syndrome (FXS) has become available on the Medicare Benefits Scheme (MBS). Screening for those three conditions is recommended for all couples prior to, or in the early stages of pregnancy. Great but what is this genetic screening all about? Why are screening? Who needs the screening? Are they for men and women? When should we do the screening? How do we organise the screening? And what happens if the results are “abnormal”?
Let’s clear up a few things. Genetic carrier screening is NOT synonymous with non-invasive prenatal testing (NIPT). Reproductive genetic carrier screening is a testing an individual or a couple’s genetic information, to find out if they have an increased chance of having a child with a genetic condition. Genetic carrier screening does not test for all types of genetic conditions.
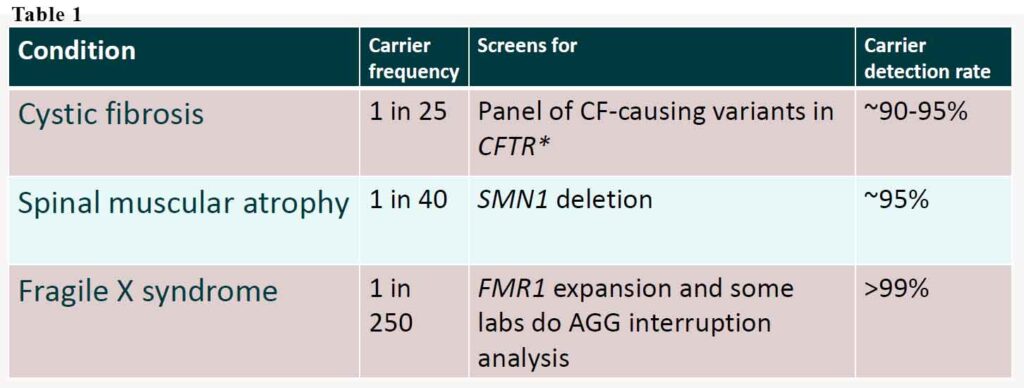
It is important to note that reproductive genetic carrier screening does not test for chromosomal conditions, such as Down syndrome. Chromosomal conditions are caused by extra or missing copies/parts of a baby’s chromosomes, not by inheriting faulty genes. The new funding is only for those 3 conditions – cystic fibrosis, spinal muscular dystrophy and fragile X syndrome. These three conditions are among the most common severe genetic conditions in childhood. Like all genetic tests, the detection rate is not 100%. Thus, the results should be referred to as low or high chance of mutation rather than negative or positive. See Table 1.
Why are we screening at all?
Cystic fibrosis is inherited in an autosomal recessive manner. It is caused by the presence of mutations in both copies (alleles) of the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) protein. It affects 1:2500 but the frequency of the gene is 1:25. It affects multiple organ systems especially respiratory and gastrointestinal. Despite advances in the multi-faceted treatment, the average lifespan is ~53 years.
Spinal muscular atrophy affects 1:10,000 but the frequency of the gene is 1:40. It is characterised in progressive muscular weakness and atrophy. It is also autosomal recessive. Spinal muscular atrophy is caused by a genetic mutation in the SMN1 gene. Muscles of lower extremities are usually affected first, followed by muscles of upper extremities, spine and neck and, in more severe cases, pulmonary and mastication muscles. Proximal muscles are usually affected earlier and to a greater degree than distal muscles. Outcomes in the natural course of the disease vary from death within a few weeks after birth in the most acute cases to normal life expectancy in the protracted SMA forms.
Fragile X syndrome affects 1:4000-6000 but the frequency of the gene is 1:250. It has traditionally been considered an X-linked dominant condition with variable expressivity and possibly reduced penetrance.[12] The likelihood of transmission depends on the parent’s gender, the X chromosome carrying the mutation, and the number of CGG repeats in the premutation. This means some males carrying the mutation has the disease while others not but can pass on the disease. Similarly, some women carrying one mutated gene may not have it because the mutated X gene is inactivated.
It is characterised by mild-to-moderate intellectual disability. The average IQ in males with FXS is under 55, while about two thirds of affected females are intellectually disabled. Physical features may include a long and narrow face, large ears, flexible fingers, and large testicles. About a third of those affected have features of autism such as problems with social interactions and delayed speech. Hyperactivity is common, and seizures occur in about 10%. Males are usually more affected than females.
- Male or female carriers may have premutation. Health impacts for people with the premutation: –
- Male carrier: fragile X associated tremor/ataxia (FXTAS) –
- Female: fragile X associated primary ovarian insufficiency (FXPOI) & FXTAS
- Risk of developing either is dependent on size of premutation
Combined together, 1 in 20 people carry a faulty gene for either CF, SMA or FXS which makes it quite common.
Who needs screening?
All women who are planning to start a family should have the discussion that genetic carrier screening is available. For women who are already pregnant by the time you read this, they should also be screened. It might also be a good time to have a conversation when women are stopping their contraception e.g. removal of Implanon or stopping the oral contraceptive pill.
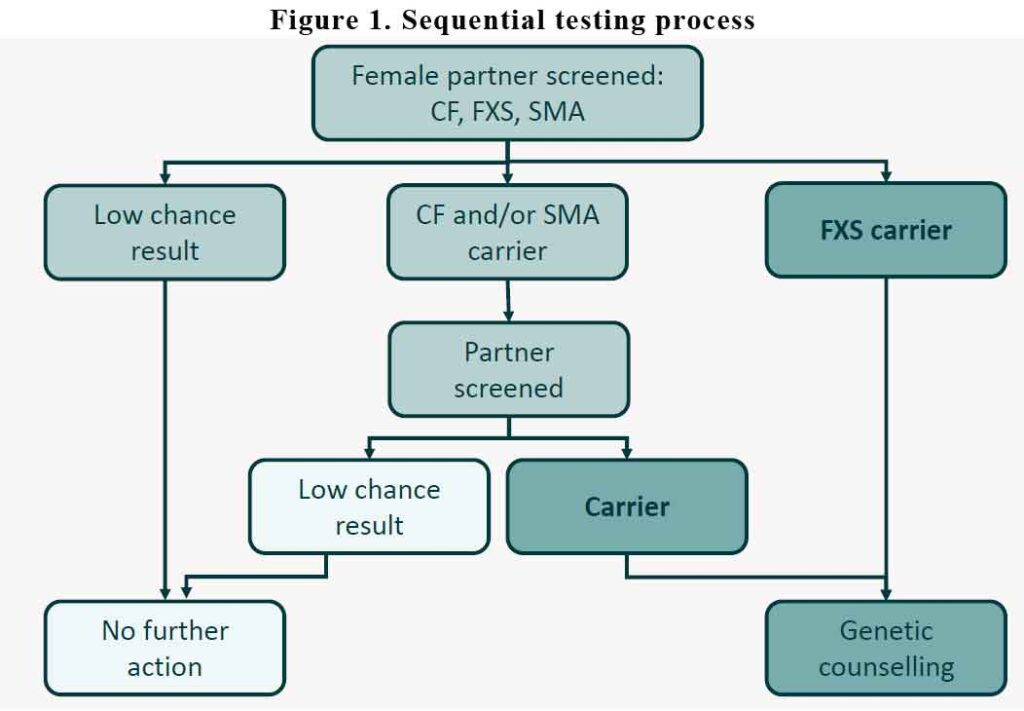
It is recommended that we screen the woman first. The MBS will not pay for screening the male partner first. The male partner is only tested if the woman tests positive for any of the 3 conditions. If the woman test “negative” for all three conditions, no further action is required. The partner doesn’t need to be tested. If she tests positive for CF and/or SMA, then the partner is tested. If the partner tests negative for CF and/or SMA, no further action is needed. In the other hand, if the woman test positive for CF/SMA and the partner also test positive for the same, then they will need referral for genetic counselling.
If the woman tests positive for FXS, then she will need to be referred for genetic counselling.
See Figure 1.
Where do patients go for screening?
Most pathology providers will be bulkbilling those tests under Medicare but each will have their own arrangements for collection. You will need to check with your provider what those arrangements are.
Larger panel of carrier screening tests
There is a larger panel of genetic carrier screening tests which are not funded by the MBS. Because the screening for CF, SMA and FXS is covered by MBS, these larger panels are expected to have reduced costs. Once again, each pathology provider will have different arrangements, costs and the array of genetic screens they cover. Douglass Hanly Moir’s list is here and Australian Clinical Labs here. Laverty Pathology also offer expanded genetic carrier screening but there is no information online.
All providers also offer information for the public on their website on genetic carrier screening. The NSW Health Centre for Genetics Screening has a fact sheet here.
In summary, in a significant proportion of children born with CF, SMA or FXS, there is no family history in either or both parents. 1:20 people carry the gene for any one of the three conditions. NIPT does not pick up these conditions and genetic carrier screening is now available to everyone under the MBS with the woman being tested first. It is recommended that we have the conversation with couples who are contemplating starting a family. It is optional for women but we need to have the conversation. It may become a medicolegal issue if they were not informed of the availability of the screening tests.
