31st May 2024, A/Prof Chee L Khoo
Renin-angiotensin system (RAS) inhibitors and SGLT2 inhibitors have been the cornerstone treatment of chronic kidney disease (CKD) for many years now. They have been shown to protect kidneys and at the same time reduce adverse cardiovascular outcomes. Finerenone was recently shown to do the same. These three agents are now the guideline-directed three pillars in the treatment of CKD. We now have the fourth pillar proven to reduce adverse kidney and cardiovascular outcomes. Semaglutide has been assessed for the prevention of kidney failure, substantial loss of kidney function, and death from kidney-related or cardiovascular causes in patients with type 2 diabetes and chronic kidney disease. The topline results of the FLOW trial were released a few months ago and the anxiously awaited full results were published last week.
Despite RAS and SGLT2 inhibitors and finerenone, many patients continue to lose kidney function and go on to renal failure or succumb to cardiovascular death. Thus, there is great interest in exploring the role of GLP1-RAs in the prevention of adverse renal outcomes.
Adults with type 2 diabetes (HbA1c ≤10%) were randomised to either semaglutide 1mg or placebo in the FLOW (Evaluate Renal Function with Semaglutide Once Weekly) trial. An 8-week dose-escalation regimen was used. Participants were eligible for inclusion in the trial if they had high-risk CKD and were receiving a stable maximal labelled dose (or the maximal dose without unacceptable side effects) of RAS inhibitors (angiotensin-converting–enzyme inhibitor or angiotensin-receptor blocker). Patients who were unable to receive RAS inhibition because of side effects were eligible for inclusion. The use of SGLT2 inhibitors and mineralocorticoid-receptor antagonists (MRAs) was permitted.
CKD was defined by an eGFR of 25 – 75 ml/min/1.73 m2 with a urinary albumin-to-creatinine ratio (uACR) of 34 – 565 mg/mmol/L if the eGFR was 50 – 75 or a uACR of 11 – 565 mg/mmol if the eGFR was 25 – 50.
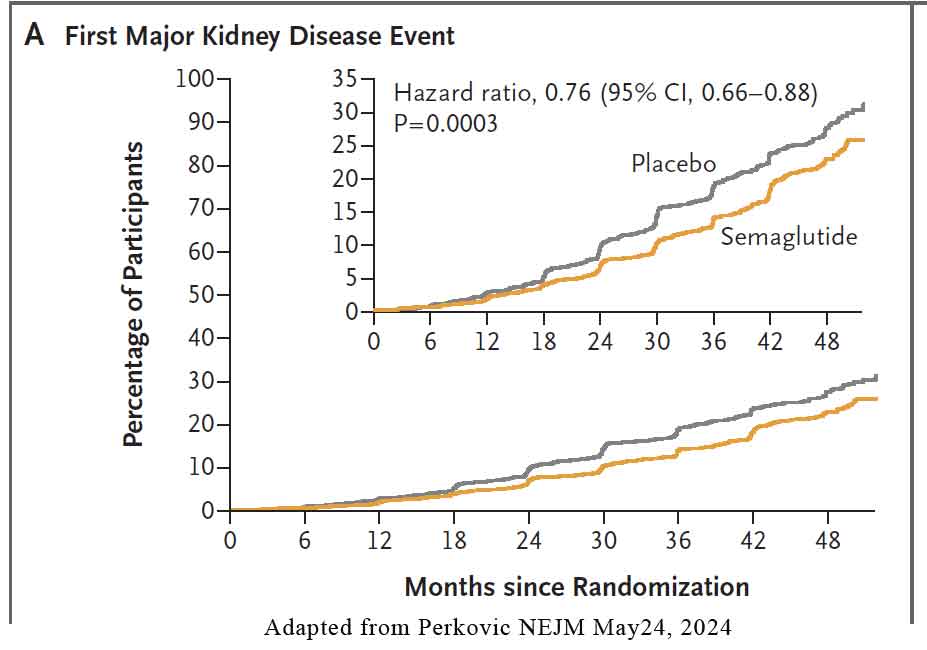
The primary outcome was major kidney disease events, a composite of onset of kidney failure (initiation of long-term dialysis, kidney transplantation, or a reduction in the eGFR to <15 sustained for ≥28 days), a sustained 50% or greater reduction in eGFR from baseline, or death from kidney-related or cardiovascular causes. See Figure below.
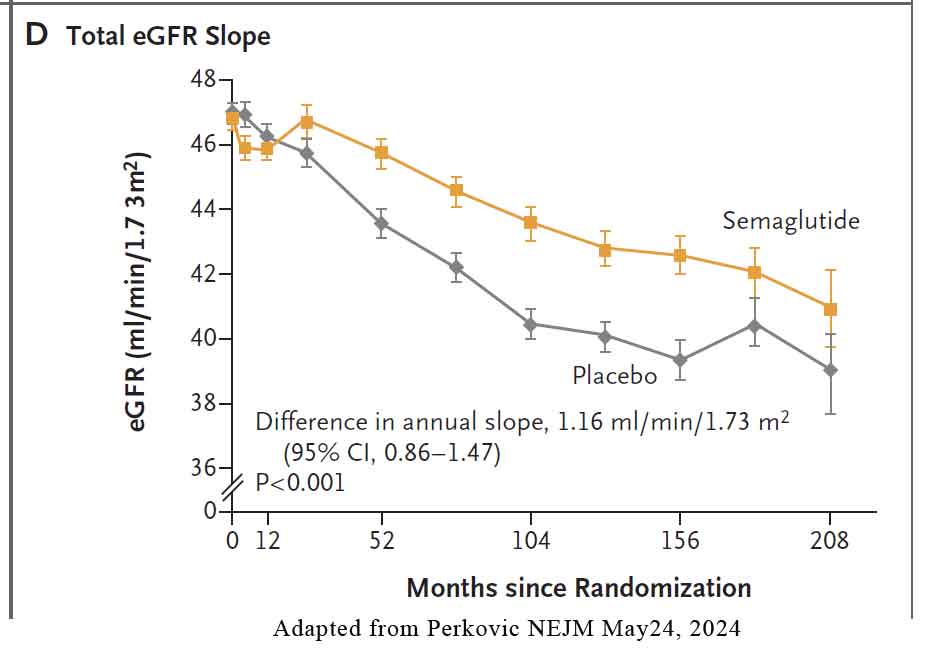
Three key confirmatory secondary outcomes were defined: 1) total eGFR slope (i.e., the annual rate of change in eGFR from randomisation to the end of the trial) 2) major cardiovascular events (a composite of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes) and 3) death from any cause.. See Figure below.
The trial was conducted at 387 sites in 28 countries. 5581 were screened and 3533 were eligible and randomised. The mean age was 66.6 years, and 1069 participants (30.3%) were women. The mean eGFR was 47.0 ml/min/1.73 m2, and the median uACR was 64 mg/mmol/L.
According to the Kidney Disease: Improving Global Outcomes (KDIGO) risk calculators, 14.68% of the participants were at very high risk for kidney disease progression, kidney failure, cardiovascular events, or death. At the time of completion of the trial, the median participant follow-up was 3.4 years. Semaglutide or placebo was permanently discontinued by 26% of participants. Adherence to the trial regimen averaging 89% of the planned time during the trial period.
The Primary Outcomes
- 24% lower relative risk of the primary outcome in the semaglutide group
- 21% lower risk with semaglutide was also observed for a composite of the kidney-specific components of the primary outcome
- 29% lower risk of cardiovascular death
- Number To Treat (NNT) over 3 years to prevent one primary-outcome event – 20
The Secondary Outcomes
- Mean annual slope of the eGFR was significantly less steep (indicating a slower decrease) in the semaglutide group
- 18% reduction of risk of major cardiovascular events in the semaglutide group
- 20% reduction of risk of death from any cause in the semaglutide group
- NTT to prevent one major cardiovascular events – 45
- NTT to prevent death from any cause – 39
Other Outcomes
- At week 104, the mean reduction in body weight was 4.10 kg greater
- Mean reduction in the HbA1c level was 0.81%
- Mean reduction in systolic blood pressure was 2.23 mm Hg greater
- Mean reduction in diastolic blood pressure was 0.78 mm Hg greater
Adverse effects
Serious adverse events were reported in fewer participants in the semaglutide group than in the placebo group (877 [49.6%] vs. 950 [53.8%])
Fewer participants in the semaglutide group were reported to have serious infections or infestations (317 [17.9%] vs. 376 [21.3%]) or serious cardiovascular disorders (273 [15.4%] vs. 319 [18.1%])
Eye disorders reported as serious adverse events were more common among participants who received v semaglutide than among those who received placebo (53 [3.0%] vs. 30 [1.7%])
Diabetic retinopathy events were similar in the two groups (504 events among 402 participants [22.8%] in the semaglutide group and 483 events among 398 participants [22.5%] in the placebo group)
In summary, in patients with type 2 diabetes and chronic kidney disease, semaglutide at a dose of 1.0 mg once weekly significantly reduced the risk of major kidney disease events (the primary outcome), by 24%. Semaglutide also reduced the risk of major cardiovascular events and death from any cause while slowing the annual loss of kidney function by a mean of 1.16 ml/min/1.73 m2. The mechanisms of kidney protection with semaglutide are likely to be multifactorial. It is likely to include the effects of GLP1-RA in reducing inflammation, oxidative stress, and fibrosis in the kidneys.
Reference:
Vlado Perkovic, Katherine R. Tuttle, Peter Rossing, et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes NEJM May 24, 2024. DOI: 10.1056/NEJMoa2403347
