28th December 2025, A/Prof Chee L Khoo
We discussed the Lancet Commission’s clinical obesity 12 months ago. Clinical obesity is obesity in the presence of a clinical disease (whether complication or co-morbidity). This is why the PBS is funding semaglutide for patients with obesity and established cardiovascular (CV) disease and not obesity on its own. There are many other “diseases” associated with obesity which will be funded by the PBS in the very near future. For now, we better explore the other incretins in SURPASS-CVOT which also has proven CV benefits as well.
SURPASS-CVOT released its topline results 6 months ago which briefly mentioned the CV benefits with tirzepatide in patients with type 2 diabetes (T2D) and established CVD. The full results were published in the NEJM just before Christmas. There were several important elements in this trial which the casual reader or reviewer may have missed.
First, this trial differs from many other CV outcome trials (CVOT) in that there was no placebo arm in the trial. It was not a tirzepatide vs placebo trial. It was tirzepatide vs dulaglutide trial. Now, dulaglutide has demonstrated CV benefits in the REWIND trial and all tirzepatide had to do was to prove that it was as good as (i.e. non-inferior to) dulaglutide with respect to CV benefits. Besides, in patients with CVD who are not on an incretin, it is no longer ethical to have them on a placebo. They had to be on another incretin therapy. In this case, it was dulaglutide. Hence, there was no placebo arm in the SURPASS-CVOT trial. We better have a look, at least, briefly, at the REWIND trial.
REWIND (2019) (1)
REWIND was conducted in a backdrop of 5 previous positive CVOT trials (ELIXA, EXSCEL, LEADER, SUSTAIN-6 and HARMONY OUTCOMES) which demonstrated the superiority of GLP-1RAs (lixisenatide, exenatide, liraglutide, semaglutide and albiglutide respectively) in reducing incident CV incidents in patients with T2D (2-6). Of note though, in the 5 trials, at baseline there was a high proportion (73-100%) of patients with known CVD. The REWIND trial, on the other hand, of the recruited 9901 people, only 31·5% were known to have previous CVD. It also had a high proportion of women (46·3%) and the participants had a mean HbA1c of 7·3%.
Over a median follow-up period of 5.4 years, weekly injections of dulaglutide reduced cardiovascular outcomes in both men and women with or without previous cardiovascular disease more compared with placebo (hazard ratio 0.88). All-cause mortality was, however, no different between the two arms. The reduced hazard ratio of dulaglutide was similar to other GLP1-RAs (LEADER – 0.87, SUSTAIN-6 0 – 0.74 and HARMONY OUTCOMES – 0.78) considering that the REWIND cohort was a mixture of patients with and without CVD.
SURPASS-CVOT (2025)
In previous clinical trials, tirzepatide have demonstrated that in addition to glycaemic and weight control, there were improvements in atherogenic lipoprotein levels, blood pressure, and kidney-related outcomes as compared with selective GLP-1 receptor agonists or other glucose-lowering agents but these trials were primarily glucose lowering trials. Specific CV outcome trials involving tirzepatide have been lacking. Till now.
Study of Tirzepatide Compared with Dulaglutide on Major Cardiovascular Events in Participants with Type 2 Diabetes (SURPASS-CVOT) recruited participants ≥ 40 years of age if they had T2D with a HbA1c between 7.0 – 10.5%, a BMI ≥ 25 and established atherosclerotic cardiovascular disease (ASCVD) in at least one vascular territory (7).
It is important to note that established ASCVD in the trial was defined as:
Coronary artery disease (CAD) with ANY of the following:
- Documented history of spontaneous MI
- ≥50% stenosis in 1 or more major coronary arteries, determined by invasive angiography
- ≥50% stenosis in 2 or more major coronary arteries, determined by computed tomography coronary angiography (CTCA)
- History of surgical or percutaneous coronary revascularization procedure
Cerebrovascular disease – ANY of the following:
- Documented history of ischaemic stroke
- Carotid arterial disease with ≥50% stenosis, documented by carotid ultrasound, magnetic resonance imaging (MRI), or angiography
- Carotid stenting or surgical revascularization
Peripheral arterial disease with EITHER of the following:
- Intermittent claudication and ankle-brachial index <0.9
- Prior nontraumatic amputation or peripheral vascular procedure (eg, stenting or surgical revascularization), due to peripheral arterial ischaemia
Participants were randomly assigned in a 1:1 ratio to receive weekly subcutaneous injections of tirzepatide at a dose adjusted up to 15 mg or dulaglutide at a dose of 1.5 mg. The primary end point was the same as in REWIND which is a composite of CV death, myocardial infarction or stroke. However, there were a number of key secondary end points:
- death from CV causes; myocardial infarction; stroke;
- death from any cause;
- a composite of death from CV causes, myocardial infarction, stroke, or coronary revascularisation;
- composite of death from CV causes or hospitalisation or urgent visit for heart failure;
- change from baseline to 36 months in the eGFR
- change from baseline in the HbA1c, body weight, and systolic blood pressure at 36 months;
- change from baseline in the triglyceride level and in the low-density lipoprotein (LDL) cholesterol level at 24 months.
At baseline
13,165 patients were included in the modified intention-to-treat population (6586 in the tirzepatide group and 6579 in the dulaglutide group). At baseline, 65.0% of the patients had coronary artery disease, 19.2% had a history of stroke, 25.3% had peripheral artery disease, 20.3% had a history of heart failure, and the mean duration of diabetes was 14.7±8.8 years. You can see that not all patients have had an event or procedure. “Established ACVD” included patients who has stenoses ≥ 50% whether they had an event or revascularisation procedure.
The mean age of the patients was 64.1 years, 29.0% were women, the mean BMI was 32.6 (+/- 5.5), the mean HbA1c was 8.4%, and the mean duration of diabetes was 14.7 years. On average, these are patients with T2D whose glucose were sub optimally controlled.
81.4% were receiving metformin, 30.6% were receiving an SGLT2 inhibitor, 21.6% were receiving a sulfonylurea, and 48.8% were receiving insulin. Recruitment started in 2020 when SGLT2 inhibitors just started to appear in the guidelines for patients at high CV risk.
Results – what did they find after 4 years of follow up?
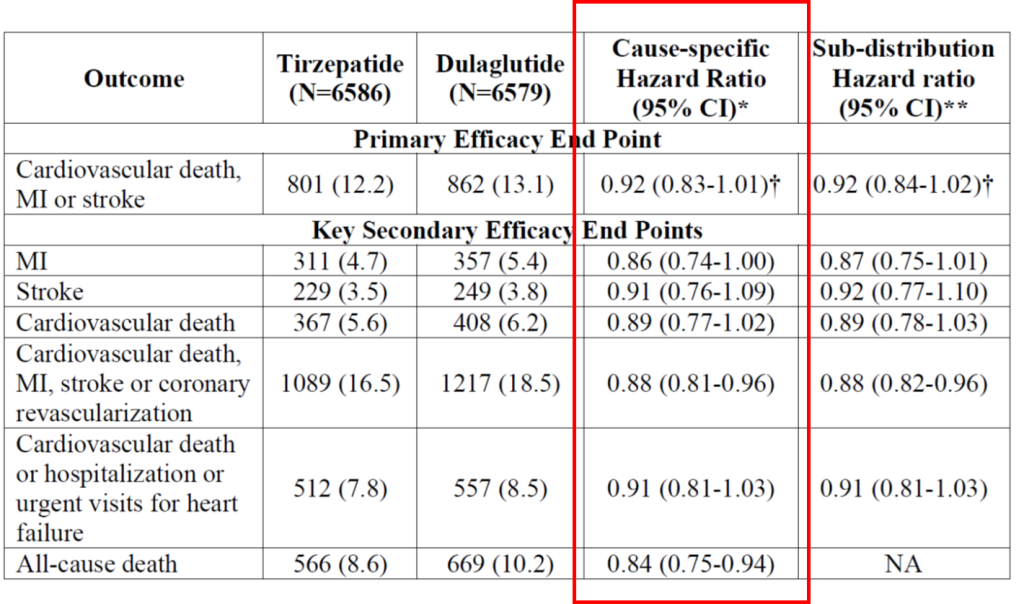
A primary end-point event (CV death, MI or stroke) occurred in 801 patients (12.2%) in the tirzepatide group and 862 (13.1%) in the dulaglutide group. This represents a hazard ratio for death from cardiovascular causes, myocardial infarction, or stroke of 0.92. The 95.3% confidence interval [CI], 0.83 to 1.01 was used. The nominal two-sided significance level was 0.047. This was above the p=0.003 value for non-inferiority. The p-value needed to be > 0.09 to be considered superior. Which means tirzepatide is as good as dulaglutide in reducing the primary end point.
When you separate the individual components in the composite outcomes out (i.e. secondary outcome measures), there was 14% reduction in MI, 9% reduction in strokes and 11% reduction in CV death. There was also a 12% reduction in cardiovascular death, MI, stroke or coronary revascularisation and 9% reduction in cardiovascular death or hospitalization or urgent visits for heart failure. There was a 16% reduction in death from any causes. See Table 1.
Metabolic changes
Overall, there was – 0.88% better reduction in HbA1c, -6.8% greater reduction in body weight, -15.6% greater reduction in triglycerides (TG) levels, 3.71 ml/min/1.73m2 less decline in eGFR and a -2.1mm Hg reduction in BP in the tirzepatide vs the dulaglutide groups. The LDL-C were reduced less by 0.02mmol/L in the tirzepatide group. Note LDL-C levels are not measured and a reduction in TG causes a paradoxical elevation of the LDL-C.
Summary
In other words, overall, tirzepatide not only improve cardiovascular events, it leads to better metabolic profile as well as better weight loss. While the PBS has now agreed to fund Wegovy for patients with obesity and established ASCVD, it shouldn’t be long before the PBS comes to the party on obesity (with or without diabetes) on the other elements of clinical obesity. In this SURPASS-CVOT trial, the mean BMI was 32.6 (+/- 5.5) which means some of the participants were not obese (BMI < 30).
References:
- Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019 Jul 13;394(10193):121-130.
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med. 2015 Dec 3;373(23):2247-57.
- Holman RR, Bethel MA, Mentz RJ, et al; EXSCEL Study Group. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2017 Sep 28;377(13):1228-1239.
- Marso SP, Daniels GH, Brown-Frandsen et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016 Jul 28;375(4):311-22.
- Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016 Nov 10;375(19):1834-1844.
- Hernandez AF, Green JB, Janmohamed S, et al; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018 Oct 27;392(10157):1519-1529.
- Nicholls SJ, Pavo I, Bhatt DL, et al; SURPASS-CVOT Investigators. Cardiovascular Outcomes with Tirzepatide versus Dulaglutide in Type 2 Diabetes. N Engl J Med. 2025 Dec 18;393(24):2409-2420.
