23rd July 2021, Dr Chee L Khoo

It’s all too confusing. And annoying. They keep releasing updates in the lay media and says “see your GP to discuss” but did not update us. You will recall that 2 weeks ago the recommendations to have the second AstraZeneca vaccination moved to 6 weeks after the first vaccination in areas with the outbreak which is pretty much all of Sydney. Straight away, efficacy numbers start popping up in the medical and lay media comparing the 6 weeks and 12 weeks interval between the jabs. Of course, patients starts bringing those number in and confront us. We need to be informed either beforehand or shortly after would be nice but there has been none. We are, once again, left to deal with patient enquiries all on our own. We better look at those numbers ourselves.
What are these efficacy numbers?
Efficacy numbers have bandied around so much that everyone seems to think that those numbers represent effectiveness of a vaccine. That’s why the public prefer the Pfizer vaccine because it has 90% plus efficacy rate versus 70% efficacy rate with the AZ vaccine. I suspect that’s the same reason many healthcare workers including doctors are jumping onto the Pfizer wagon too (I know, the clot issue is another reason). Those general efficacy numbers came from the Phase 3 clinical trials of the two vaccines but real world data shows both to be > 90% efficacious in reducing symptomatic infection. We better define a few terms before we get carried away by these fancy numbers.
Efficacy numbers
The Phase 3 clinical trials compared vaccinated subjects with unvaccinated control. The primary outcome was symptomatic disease confirmed by swabs. Patients were sent away and only contact the research centre when they have symptoms and a swab is organised to confirm. Patients who did not have symptoms were not assessed. So, we do not have data on asymptomatic carriers. Obviously, some patients who had symptoms and tested positive on swabs had severe disease or died and data on those were collected in the trials. So, we have efficacy on hospitalisation or death.
What about transmission?
The Phase 3 trials were conceived early last year and started in April 2020. Back then, we did not know about asymptomatic transmission. Unfortunately, to be able to assess for transmission, we need to include swabbing asymptomatic subjects which was only done in a small sample of subjects in the AZ trials. The numbers were encouraging but were not powered statistically. It doesn’t mean the vaccines do not prevent transmission. It just that because it wasn’t looked for in the trials, no one can claim reduction in transmission. You only have to look at the numbers in UK and US before and after mass vaccination to see that vaccination do reduce transmission.
Sub analysis of the original AZ trial was conducted to explore the efficacy of the vaccine stratified by the timing of the second dose. It is worth digesting those numbers as it will be helpful to discuss with the worried patients coming in earlier for their second jab.
Efficacy of AZ ChAdOx1 nCoV-19 vaccine
Overall, there were 332 cases of primary symptomatic COVID-19 occurring more than 14 days after a booster dose, 84 (1·0%) in the 8597 participants in the ChAdOx1 nCoV-19 group and 248 (2·9%) in the 8581 participants in the control group, with overall efficacy of 66·7%. Of the 332 cases, 271 occurred in patients who had two standard doses while 61 occurred in patients who had one half dose followed by one standard dose. The efficacy rate for those who had two standard doses, which is what we advocate in Australia, were 63.1% while the subgroup of patients who received a half dose followed by standard dose had an efficacy rate of 80.7%.
The time interval between 1st and 2nd dose
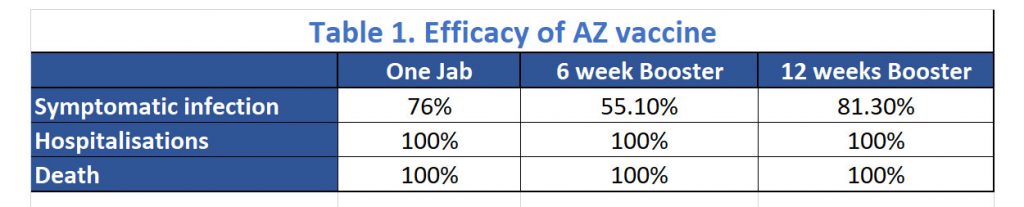
Let’s have a look at the efficacies against primary symptomatic infection in patients after one dose and in patients who had the second dose 6 weeks and 12 weeks apart. A single standard dose of vaccine provided protection against primary symptomatic COVID-19 in the first 90 days with an efficacy of 76·0% (95% CI 59·3 to 85·9) but was not efficacious against asymptomatic infection over the same time period. There were no evidence of waning of protection in the first 3 months after vaccination.
Vaccine efficacy after the second dose was higher in those with a longer prime-boost interval, reaching 81·3% in those with a dosing interval of 12 weeks or more versus 55·1% in those with an interval of less than 6 weeks. See Table 1. They identified a higher efficacy in a subgroup analysis of those who received the low dose plus standard dose regimen. However, it was decided that the two standard doses regimen is preferred operationally because it is more straightforward to deliver the same vaccine for both doses and because there are more immunogenicity and efficacy data to support its use.
Practical implications of this sub-analysis
While the head-line numbers would suggest that an earlier second dose may have lower efficacy rate against symptomatic disease, the efficacy against hospitalisation and death is unchanged by the varying intervals of the second dose. Interestingly, the 100% efficacy against hospitalisation was seen from 22 days after the first dose.
During an outbreak like the one sweeping through Greater Sydney now, it is probably wise to bring forward the second dose. Efficacy against symptomatic disease may be lower but protection against severe disease requiring hospitalisation is maintained. We can safely reassure our patients when we bring forward their second jab.
Reference
Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Clutterbuck EA, Collins AM, Cutland CL, Darton TC, Dheda K, Dold C, Duncan CJA, Emary KRW, Ewer KJ, Flaxman A, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Galiza E, Goodman AL, Green CM, Green CA, Greenland M, Hill C, Hill HC, Hirsch I, Izu A, Jenkin D, Joe CCD, Kerridge S, Koen A, Kwatra G, Lazarus R, Libri V, Lillie PJ, Marchevsky NG, Marshall RP, Mendes AVA, Milan EP, Minassian AM, McGregor A, Mujadidi YF, Nana A, Padayachee SD, Phillips DJ, Pittella A, Plested E, Pollock KM, Ramasamy MN, Ritchie AJ, Robinson H, Schwarzbold AV, Smith A, Song R, Snape MD, Sprinz E, Sutherland RK, Thomson EC, Török ME, Toshner M, Turner DPJ, Vekemans J, Villafana TL, White T, Williams CJ, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ; Oxford COVID Vaccine Trial Group. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021 Mar 6;397(10277):881-891. doi: 10.1016/S0140-6736(21)00432-3. Epub 2021 Feb 19. Erratum in: Lancet. 2021 Mar 6;397(10277):880. PMID: 33617777; PMCID: PMC7894131.
